Early Detection,
Better Outcomes.
Guided Therapeutics, Inc. (OTC: QB GTHP) is committed to developing groundbreaking medical devices using our patented biophotonic technology to detect diseases earlier, more accurately, and more efficiently than other tests.
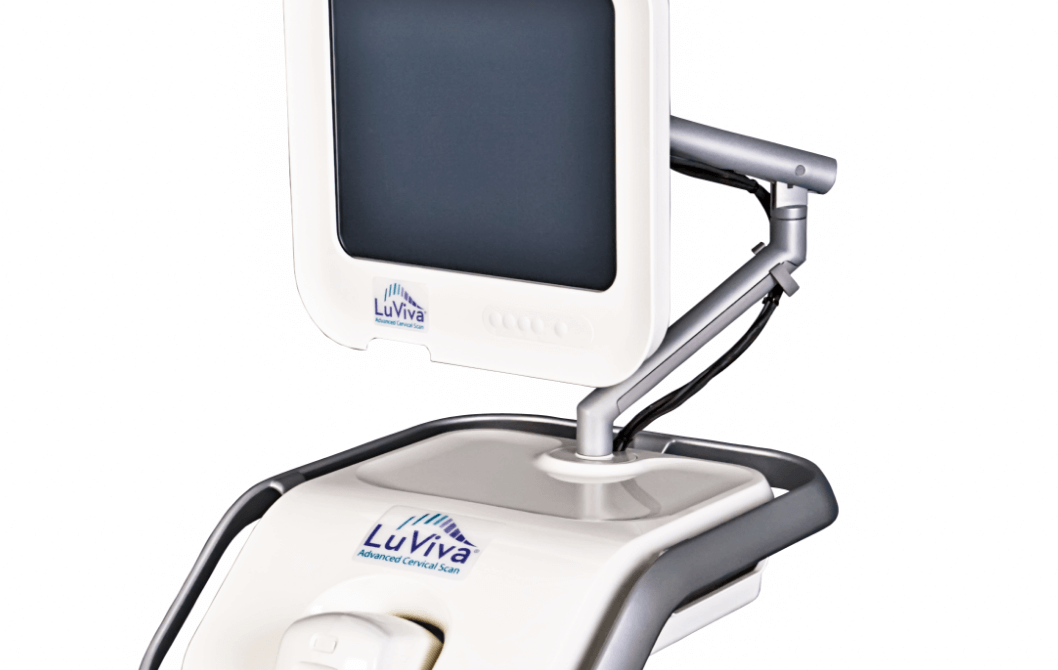
Product Information
Our products are designed to improve the patient’s experience, provide the physician with more powerful tools, improve the efficiency of the healthcare system and increase shareholder value.
How we are making
a difference globally
Our products are designed to:
Improve the patient’s experience.
Provide the physician with more powerful tools.
Improve the efficiency of the healthcare system.
Technology
Our patented technology, which features our flagship product the LuViva® Advanced Cervical Scan, uses biophotonics to detect both physical and chemical changes in tissues that are early signs of cancer.
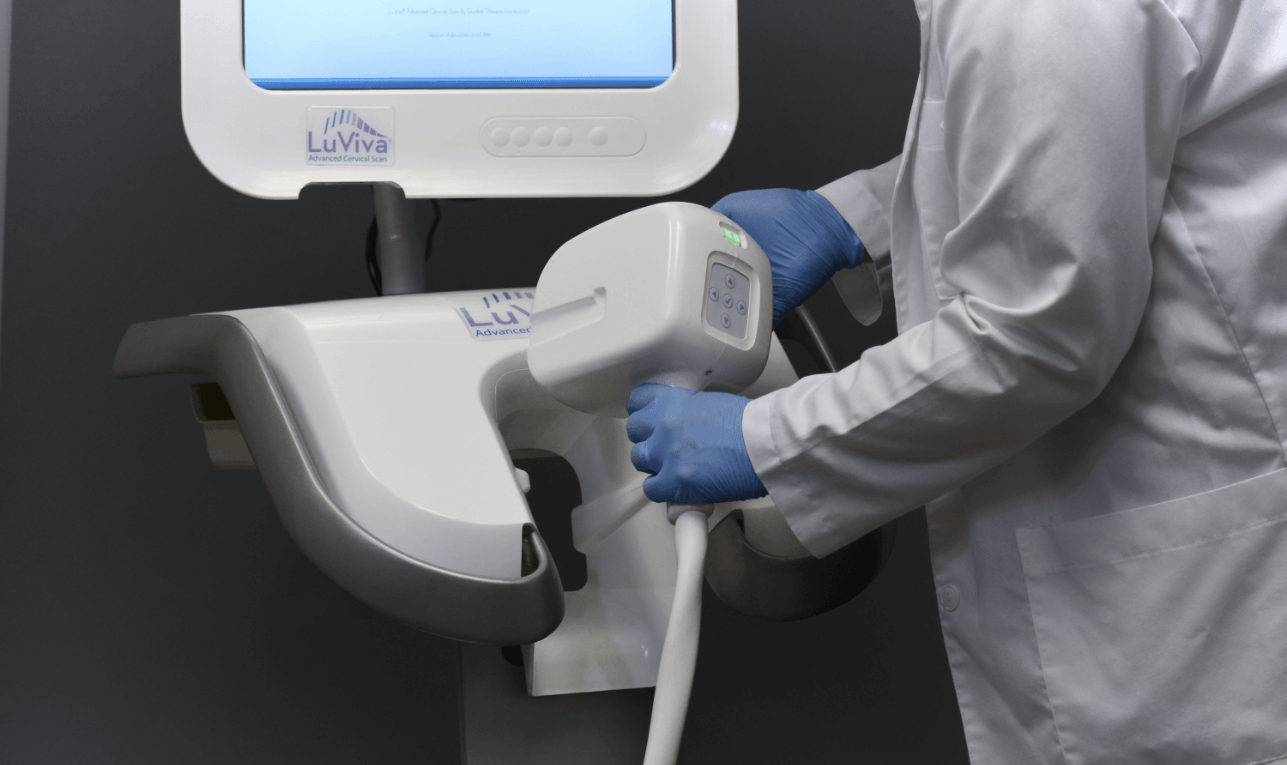

Investors
Cancer screening is a compelling market opportunity proven to save lives. As a pioneering company in this space, GTI represents an excellent investment.
Join our network of Investors
We are actively seeking new investors to join our network. We provide full transparency into our organization and would love to have you join.
We’re international and expanding. Read about Guided Therapeutics
Recent Press Releases
Guided Therapeutics Announces Filing of Application for NMPA Approval to Market and Sell LuViva in China
October 22, 2024
Guided Therapeutics’ Announces Data from Chinese NMPA Clinical Study Signed Off by All Four Clinical Sites with Better Than Expected Results
July 10, 2024
Leading Cervical Cancer Doctors Support Approval of LuViva in China Based on Preliminary Review of Clinical Trial Results
February 20. 2024
GTI Provides Update on Start of Clinical Trial for US FDA Approval
May 01, 2023
